Radioligand Therapy Market To Reach $15.80B by 2033 | DataM Intelligence | Driving Cancer Innovation
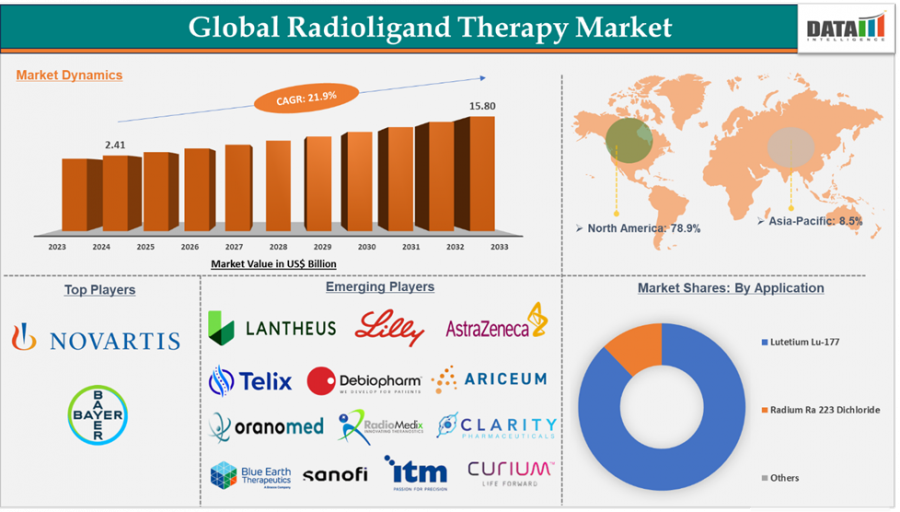
Radioligand Therapy Market is set to grow rapidly, rising from $2.41B in 2024 to $15.80B by 2033, driven by advanced cancer treatments and targeted therapies.
AUSTIN, TX, UNITED STATES, June 13, 2025 /EINPresswire.com/ -- Radioligand Therapy Market Overview (2025)
The Radioligand Therapy Market Size was valued at USD 2.41 billion in 2024 and is projected to grow significantly, reaching approximately USD 15.80 billion by 2033. This growth reflects a strong compound annual growth rate (CAGR) of 21.9% over the forecast period from 2025 to 2033.
To Download Sample Report: https://datamintelligence.com/download-sample/radioligand-therapy-market
Latest Industry Developments
In May 2025, Blue Earth Therapeutics began enrolling patients in a Phase 2 clinical trial for its investigational radioligand therapy, a lutetium-labeled, PSMA-targeted radio hybrid treatment. The study’s key goals include evaluating how many patients experience a ≥50% drop in PSA levels, monitoring radiographic progression-free survival, and assessing overall patient safety.
In March 2025, Novartis AG received FDA approval for the expanded use of Pluvicto, allowing it to be prescribed earlier in the treatment cycle before chemotherapy for patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). This approval is expected to triple the eligible patient population for Pluvicto, significantly widening its clinical impact.
Key Regional Insights
North America
North America accounts for the largest share of the market. With high awareness about advanced cancer treatments and strong insurance support, the U.S. continues to lead clinical trials and commercial product approvals. Increasing investments by pharmaceutical giants and collaborations between biotech startups and cancer research institutions are fostering faster innovation cycles.
Europe
Countries like Germany, Switzerland, and the UK are at the forefront of adopting radioligand therapies. National cancer care strategies are being aligned to include newer precision treatments, supported by favorable reimbursement models. The European Medicines Agency (EMA) has been actively reviewing and approving new drugs, which is further stimulating market growth in this region.
Asia-Pacific
The Asia-Pacific region is catching up rapidly. Countries like Japan, South Korea, and Australia are investing heavily in cancer research and diagnostics. The demand for more effective late-stage cancer treatments is encouraging healthcare systems to integrate radioligand therapy into mainstream oncology care. As regulatory frameworks evolve, the region is becoming an attractive market for global players.
Competitive Landscape
Novartis AG
Bayer AG
Emerging Players
Eli Lilly and Company.
Lantheus.
Curium
AstraZeneca
ITM Isotope Technologies Munich SE
Telix Pharmaceuticals Limited.
Oranomed
Sanofi
RadioMedix, Inc.
Clarity Pharmaceuticals
Ariceum Therapeutics
Blue Earth Therapeutics
Market Segmentation:
By Type: Lutetium Lu-177, Radium Ra 223 Dichloride, Others
By Target: Prostate-specific Membrane Antigen (PSMA), Somatostatin Receptor (SSTR)), By Application (Prostate Cancer, Gastroenteropancreatic Neuroendocrine Tumours (GEP-NETs), Others
By Region: North America, Latin America, Europe, Asia Pacific, Middle East and Africa
Recent Trends:
A noticeable uptick in clinical trial approvals for new radioligand therapies.
Rising adoption of theranostics, combining diagnostic imaging with therapeutic radioisotopes.
Increase in hospital infrastructure for nuclear medicine units worldwide.
Growing preference for non-invasive and highly targeted treatments among patients and physicians.
Latest News from the USA
In Q2 2025, several U.S.-based cancer centers announced the rollout of next-generation radioligand therapies targeting metastatic prostate cancer. This move follows recent FDA approvals of expanded indications for drugs already on the market, such as PSMA-targeted radiopharmaceuticals.
Moreover, major pharmaceutical companies headquartered in the U.S. are investing in large-scale manufacturing facilities to improve domestic production and reduce reliance on imported radioisotopes. These investments are expected to enhance supply chain reliability and lower costs for patients.
In a landmark event, a U.S. biotech firm recently launched the first human trial for a multi-target radioligand therapy, which aims to simultaneously attack prostate and bone metastases. The trial is gaining attention for its potential to revolutionize how metastatic cancers are treated in the U.S.
Latest News from Japan
In 2025, Japan’s Ministry of Health, Labour and Welfare granted approval for the country’s first homegrown radioligand therapy, developed through a partnership between a leading academic institution and a Tokyo-based biotech startup. This marks a pivotal moment, as Japan aims to localize production and reduce dependence on imported therapies.
Furthermore, Japanese researchers have announced the success of a pilot program using radioligand therapy for rare neuroendocrine tumors, achieving encouraging patient outcomes with limited side effects. The findings were presented at an international oncology summit in Osaka, where Japan's leadership in nuclear medicine technology was acknowledged.
The country is also making strategic efforts to train more nuclear medicine professionals and is planning to expand radiopharmacy units in public hospitals by 2026. These steps are being taken in response to the increasing demand for precision oncology solutions among its aging population.
Conclusion
The Radioligand Therapy Market is moving steadily toward becoming a cornerstone of modern cancer treatment. With strong support from governments, growing patient awareness, and continuous pharmaceutical innovation, this sector is expected to witness significant breakthroughs in the years ahead.
The U.S. is pushing the frontiers through advanced trials and infrastructure investments, while Japan is carving out a strong domestic capability for research and delivery. As the global healthcare ecosystem increasingly prioritizes targeted and minimally invasive treatments, radioligand therapy is poised to become a standard of care for several hard-to-treat cancers worldwide.
Here are the Latest Experts Related Reports By DataM Intelligence
Gastroparesis Treatment Market Size 2025-2033
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
Sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release